![]() The Pacific War Online Encyclopedia
The Pacific War Online Encyclopedia
|
| Previous: Antiaircraft Cruisers (CLAA) | Table of Contents | Next: Antioch |
Malaria is a debilitating and
sometimes fatal disease caused by parasitic microorganisms of the
genus Plasmodium. Once
widespread from the tropics to as far north as Britain, wherever
standing water provided a suitable breeding ground for the
mosquitoes responsible for its transmission, malaria had been
partially brought under control in the United States and Europe
by the time of the Pacific War. However, it was still widespread
throughout the southern and southwest Pacific and southeast Asia,
where it was often the single greatest cause of military casualties.
Malaria could be treated with quinine, but 90% of the world's
supply of this vital commodity was grown on Java, which was lost to the Japanese in March 1942. This gave
added urgency to the search for new antimalarial drugs.
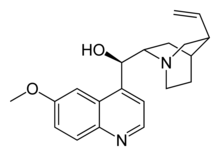
Quinine is an alkaloid obtained as an extract from
cinchona bark. It has an intensely bitter taste and is used to
flavor certain alcoholic
beverages. It also relieves muscle cramps. But by far its
most important use in 1941 was as an anti-malarial drug. A dose of
10 grains (0.65 grams) a day was administered as a prophylactic to
prevent contracting the disease, while 30 grains (1.94 grams) per
day was the appropriate dose for treating active malaria.
Quinine destroys the malaria parasites in the bloodstream, most likely by interfering with the parasites' ability to convert toxic heme from digested hemoglobin to a harmless crystalline form (hemozoin). Heme then builds up in the parasite cells and poisons them. Quinine is an effective cure for falciparum malaria, the most common and serious form of the disease. It does not fully cure the milder relapsing strain of malaria (vivax malaria), because only the parasites in the bloodstream are poisoned and not the parasites that hibernate in the liver, but quinine is effective in suppressing the symptoms of vivax malaria.
The chinchona from which quinine is obtained is a small tree (around 30' or 10m in height), distantly related to the coffee bush, that is native to the tropical Andes mountains of South America. The powdered bark had been used by native healers even before malaria was introduced into the New World by Old World explorers, but its effectiveness against malaria was first recorded by the Augustinian missionary Father Antonio de la Calanca in 1633. (The claim that chinchona was first successfully used by Europeans to save the life of the Countess of Chinchón is apparently a myth.) In spite of efforts by Bolivia to maintain a lucrative monopoly on the drug by banning the export of seeds, the British smuggled out some 100,000 seeds in 1860, but these proved to be of low-yield strains. In 1865, the Dutch were more successful, and soon had their own near-monopoly, administered by the Amsterdam-based Kina Bureau. By late 1941 there were some 37,000 acres (15,000 hectares) of chinchona plantations on Java producing about 20 million pounds (9 million kilograms) of bark a year. Bark was harvested from the branches and roots of six- to eight-year-old trees grown from cuttings of fast-growing high-yield varieties, and the quinine extracted as the sulfate.
Following the loss of Java, the Allies were forced to rely on South American production, but this was very small and could not be ramped up quickly. A procedure for manufacturing synthetic quinine was developed in 1944, but until then the Allies had to rely on other synthetic antimalarials, of which the most important was Atabrine (quinacrine, also sold as Atebrin).
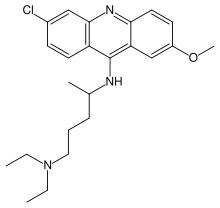
The development of Atabrine took its impetus from the
defeat of Germany in the
First World War. With the loss of its African colonies, Germany
became dependent on the Dutch for quinine, a situation which the
Germans found unacceptable. I.G. Farbenindustrie began a search
for a synthetic antimalarial that drew on the theories of Paul
Ehrlich. Ehrlich had noted that chemical dyes applied to
microscope slides often selectively stained only certain tissues
or microorganisms. He theorized that dyes might be found that
would be selectively taken up by disease-causing microorganisms in
the human body. If these dyes also poisoned the microorganisms,
then they might cure disease without harming the human patient.
Ehrlich's program led to the discovery of such important families
of drugs as Salvarsan and the sulpha drugs.
It was known that methylene blue, an intense blue dye
derived from coal tar, had significant antimalarial activity.
Using canaries infected with avian malaria as test specimens,
Wilhelm Roehl of I.G. Farbenindustrie began looking for compounds
related to methylene blue that were less intensely colored (to be
more acceptable to patients) and more effective. The first
discovery, Plasmoquine, was effective but had unpleasant side
effects. However, it was the only antimalarial on the market that
fully cured the relapses of vivax malaria. By the late 1920s,
research had shifted to acridine dyes and the use of Java sparrows
as test animals, and in 1932 I.G. Farbenindustrie published the
first reports of a new compound, Plasmoquine E, later renamed
Erion and then Atabrine, which was highly effective against
malaria.This was soon marketed throughout the world.
Atabrine came in the form of little yellow pills.
Intensely bitter, like quinine, Atabrine was still new enough in
1942 that the appropriate dose had not been completely worked out.
Overdose caused jaundice, headache, vomiting, and (in a few cases)
psychosis. I.G. Farbenindustrie's American distributor, Winthrop
Chemical Corporation, was careful to avoid mentioning these
unpleasant side effects in its literature, and in any case
Atabrine was the best substitute available for quinine. Production
in the United States was greatly expanded, from five million
tablets a year in 1939 to 3,500 million tablets in 1944, and
Winthrop reduced the selling price to 10% of its original value.
British production, by Imperial Chemical Industries, went from
practically zero in 1939 to 2,000 million tablets in 1943. ICI had
also produced some 32 million tablets of Plasmoquine by 1942.
Even at appropriate doses, Atabrine temporarily turned the skin bright yellow. A common (and unfounded) rumor was that the pills caused sterility, a belief that was exploited by Japanese propaganda leaflets. The troops often tried to avoid taking the pill, so medical officers had to supervise administration. In at least one theater, officers were threatened with relief for cause if their troops failed to make proper use of Atabrine and mosquito netting (Weina 1998) :
Sir William Slim's method for ensuring command responsibility was to organize surprise checks on units to assess the compliance with Atabrine discipline: "If the overall result was less than ninety-five per cent positive, I sacked the commanding officer. I only had to sack three; by then the rest had got my meaning." With regard to these events, Cantlie stated, "When for the first time in history a combatant officer was considered unfit to command a unit on the grounds that he had allowed his men to become ineffective through disease, a new day in military medicine dawned. The clouds of forgetfulness must not be allowed to overshadow the brightness of that day."
Medical officers at Cape Gloucester tried a different tack, posting roadside signs featuring pictures of nude women with captions like "Two Reasons Why You Should take Atabrine." The Marines responded with their own signs, displaying taunts like "Why Wear A Truss? Take Atabrine." Sometimes a grimmer approach was tried:

National Museum of Health and Medicine.
Via j-walkblog.com
Atabrine was not infallible. Besides being ineffective
at preventing relapses of vivax malaria, it was not fully
effective against all strains of falciparum malaria.
Atabrine-resistant strains of malaria had made their appearance in
the Sepik River region of New
Guinea by the time the war ended, and may have begun to
emerge in Burma as well.
Atabrine was known to the Japanese, who produced it in small amounts to supplement their supplies of quinine.
Research on new antimalarials in the United States became the biological counterpart of the Manhattan Project and the Radiation Laboratory. Much of the basic research was conducted at Johns Hopkins University, where new methods of monitoring plasma drug concentrations at various doses were worked out. However, some of the best clinical studies were carried out by the Australian research unit at Cairns. Led by Brigadier Niel Fairley, the Australian researchers flew in 20,000 Anopheles mosquito larvae from New Guinea every week to feed on malaria carriers and then be used to infect volunteers. The Australians determined that sporozoites from infected mosquitoes disappeared from the bloodstream in 30 to 60 minutes, although it was not until 1948 that it was established that the sporozoites disappeared because they invaded the liver. It was found that a dose of 100 mg of Atabrine per day was adequate under field conditions to cure active falciparum malaria or suppress the symptoms of vivax malaria. (Unfortunately, like quinine, Atabrine did not destroy the malaria parasite in the liver, but only in the bloodstream.) This was a third the dose originally recommended, which was based on the dose required for quinine.
The antimalarial program screened more than 13,000 compounds, of which around 100 were tested at the clinical level, and it laid the groundwork for development of important drugs such as chloroquine and proguanil. The path to chloroquine began in 1934, when the I.G. Farbenindustrie team discovered a powerful antimalarial which was patented as Resochin. This was considered too toxic to be a useful drug, based on animal studies, but Bayer chemists developed a derivative, Sontochin, which was also potent against malaria but thought to be less toxic to humans. Some of the supply was given to the French for testing in Tunisia, and this fell into the hands of the Allies after the Torch invasion in 1942. Nothing further was done with the compound until 1943, due in part to a mistaken analysis of its chemical structure. However, a derivative, SN-7618, was tested in 1944 and found to be both highly effective against malaria and much less toxic than Sontochin. The new compound turned out to be chemically identical to to the original Resochin. Though relatively toxic to dogs, which is why it was dropped by the Germans, it proved much less toxic to primates. SN-7618 or Resochin, subsequently renamed chloroquine, was colorless, had far fewer side effects than Atabrine, and was just as effective. Unfortunately, although Fairley's Australians subsequently worked out the correct dosing, chloroquine was not ready for mass production in time for use in the Pacific War.
A case can be made that most effective chemical agent against malaria was DDT, which is relatively nontoxic to mammals but was deadly to malaria-carrying mosquitoes. It was liberally dusted over base camps and even the front lines.
References
herbs2000,com
(accessed 2013-2-15)
National Institutes of Health (accessed 2008-6-24)
Oregon Health and Science University (accessed 2011-6-17)
Weina, Military Medicine (September 1998; accessed 2008-6-24)
The Pacific War Online Encyclopedia © 2006, 2008, 2011, 2013, 2016 by Kent G. Budge. Index